FDA REMS Programs: Five Major Components for Compliance

The US Food and Drug Administration (FDA) Amendments Act of 2007 authorized the FDA to require a Risk Evaluation and Mitigation Strategy (REMS) Program. The REMS program is required if it is determined that specific safety measures are needed to ensure that a drug’s benefits outweigh the risks, either during initial product review or at any point in the post-marketing period.
FDA REMS programs may be required for a single drug or broadly across a general classification of drugs, which share risk-benefit components. Prior to 2007, clinical trial sponsors were issued a Complete Response Letter (CRL) which left potential drugs in deferred regulatory approval indefinitely, until additional safety data could be obtained.
Pre-approval REMS requirements have provided avenues to obtain the additional safety data which may trigger an FDA REMS program if not included with the application. Post-approval REMS are typically requested when new safety information determines necessity to ascertain if the drug benefits outweigh the safety risks.
There are currently five basic components that must be included within an FDA REMS program.
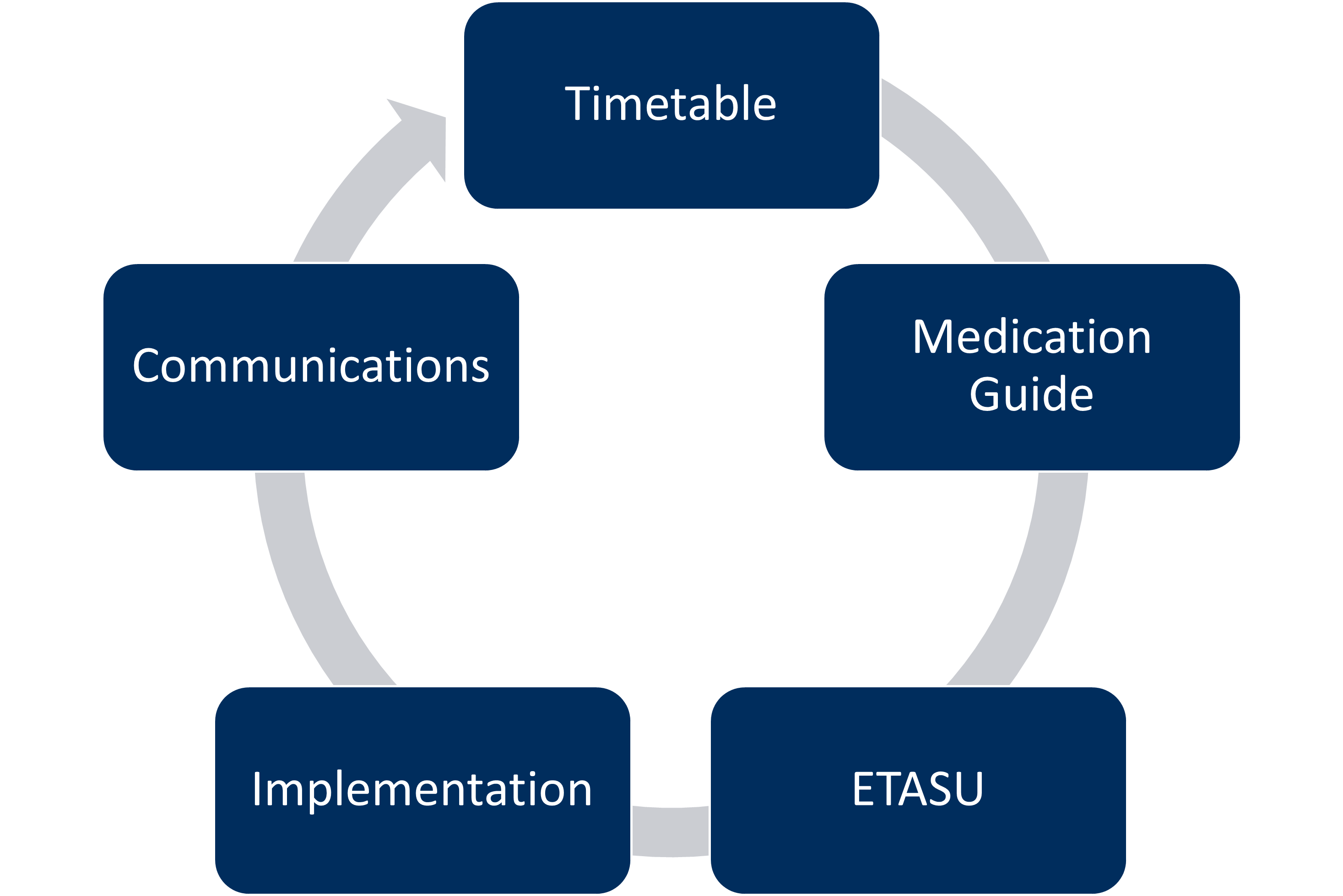
Component 1: Timetable for Submission of Assessments
The Timetable for Submission of Assessments lays out when Sponsors must assess how well the REMS is working and the FDA reporting requirements. Clinical trial sponsors must include specific goals as evaluation metrics.
These assessments compare the results of the program to the initial goals at 18 months, three years, and seven years.
Component 2: Medication Guide
A medication guide may be required if it could help avoid Serious Adverse Events (SAEs). The purpose of the medication guide in an FDA REMS program is to inform patients about serious side effects before they decide to use a drug.
Medication guides are intended to be read and understood by the patient with adherence to prescribing and dosing, which is essential to the drug’s effectiveness measures.
Component 3: Elements to Assure Safe Use (ETASU)
Many Elements to Assure Safe Use (ETASU) requirements focus on actions that are performed prior to the drug being dispensed or prescribed to a patient, such as certifications and dispense only requirements. Other elements, such as patient monitoring, may be required for the duration of the treatment.
The certification ensures that the healthcare providers are aware of any serious risks and know how to look for and control potential adverse events associated with the drug. FDA REMS programs can require a pharmacist or anyone else who dispenses the drug to be certified – not just the prescribing physician.
Component 4: Implementation System
An implementation system to track certain ETASU elements may be required. Sponsors are tasked to ensure reasonable steps to monitor and evaluate those in the healthcare system who are responsible for implementing necessary safe use measures.
Component 5: Communication Plan
A communication plan serves to educate and raise awareness of the risks associated with the drug’s use. They are similar to medication guides, except are directed to healthcare providers instead of patients.
In addition to risk awareness education, communication plans alert healthcare providers of any changes in the FDA REMS program so that they can properly care for their patients taking a specific medication. Communication plans can be letters sent directly to healthcare providers and often include distributions of informational materials to professional societies.
Consider this when implementing a REMS Program
The FDA can require a REMS program if they determine that safety measures are needed beyond drug labeling. Sponsors are responsible for developing their REMS program for a specific drug or if the drug falls within a class of drugs which require REMS. The safety measures associated with each REMS are specific to the unique risks involved.
No two REMS programs are identical.
There are commonalities for the successful implementation and management of an FDA REMS program. REMS are not designed to mitigate all adverse events of a medication. The focus is on preventing, monitoring, and/or managing a specific serious risk by educating prescribers, pharmacists, and patients to reduce the frequency or severity of the adverse event occurrence.
It is not always easy to implement REMS programs, as they require specific components to remain in compliance and avoid FDA observations. The resources, time, and cost of an FDA REMS program must be evaluated prior to implementation.
Partnerships with FDA REMS program providers, like clinical research organizations (CROs), can alleviate some of these burdens from the pharmaceutical companies and place the workload on the organizations, with expertise, in delivering the successful components in compliance with FDA REMS requirements.
For questions on implementing these processes and helping avoid FDA REMS Observations, Please click here to connect with the right resource to respond.
Authored by: Kelly Chain; Senior Manager, Quality and Compliance.