Putting the Action in Diversity Action Plans and the Real-Time Data Visualization Technology Needed to Ensure It Happens

The goal of the FDA’s June 2024 Draft guidance on Diversity Action Plans is to increase clinical trial enrollment among populations historically underrepresented in biomedical research through the use of a specific tool: the Diversity Action Plan. The recommendations on the structure and content of these plans represent a systematic approach to the longstanding and complex challenge of collecting clinical trial data generalizable to the total population that may benefit from a given treatment.
Consistent with the importance of this goal, these plans will be legally required under FDORA Section 3601 which mandates both submission of Diversity Action Plans and ongoing monitoring of related enrollment targets. These rules will go into effect 180 days after the FDA publishes their final guidance on the topic.
For more details on this guidance please see this article
Given the complexity and need for advance planning associated with large pivotal trials, as well longer-term considerations for evaluation of data across studies, sponsors should begin considering how these guidelines might affect their clinical development plans now.
One area for proactive planning is the creation and implementation of monitoring plans to track enrollment goals and allow responsiveness to these data. Good monitoring plans will be essential to the effective execution of Diversity Action Plans. To illustrate this, view Figure 1 below, which outlines steps in the planning and execution of these plans including characterization of the patient population, creation and filing of the Diversity Action Plan, monitoring and updating of the plan, and ultimately justifying enrollment.
The heart of the process lies in monitoring and plan updating. Without these iterative steps, responsiveness to enrollment deficits during clinical trials will not be possible. Thus, determining best practices for these steps will be crucial in meeting the ultimate goal of increasing clinical trial participation in underrepresented populations.
It will be particularly important for sponsors to develop best practices to track and respond to factors related to the FDA’s expanded definition of diversity, which include not only evaluation of different age groups, sexes, and racial and ethnic demographic characteristics but also “health disparities and differential access to health care and clinical studies that may that occur based on other factors, including but not limited to geographic location, gender identity, sexual orientation, socioeconomic status (SES), physical and mental disabilities, pregnancy status, lactation status, and co-morbidity (FDA, June 2024).” Owing to the complexity of these variables, data visualization software is expected to play a key role in effective and timely monitoring enrollment objectives facilitating timely enrollment-improvement actions. Below, we describe data visualization tools and practices that will help sponsors to put the action in their Diversity Action Plans from first to last patient enrolled.
Figure 1: Key Steps in the Planning and Execution of Diversity Action Plans
A Tech-Enabled/Enhanced Approach – Diversity Dashboards Overview
In any clinical trial, keeping track of enrollment as a study progresses is a challenge. Between subjects being randomized, tracking dispositions, and managing communication between sites; it can be easy to lose track of diversity metrics. MMS Datacise® dashboards seek to revolutionize how we track, view, and interact with trail diversity.
These Datacise diversity dashboards are designed to present diversity data in near real-time in a fully interactive model. The dashboards are optimized around CDASH/SDTM standardized data but are flexible enough to accommodate nearly any data source. Most commonly this is refreshed on a daily or hourly basis to ensure that the most recent data is always present for review.
Pictured below are three examples of how Diversity data be presented:
- Study Overview,
- Ethnicity, and
- Sex.
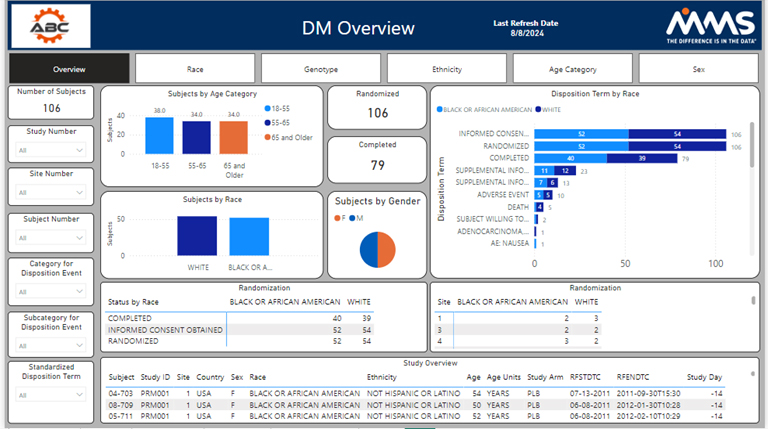
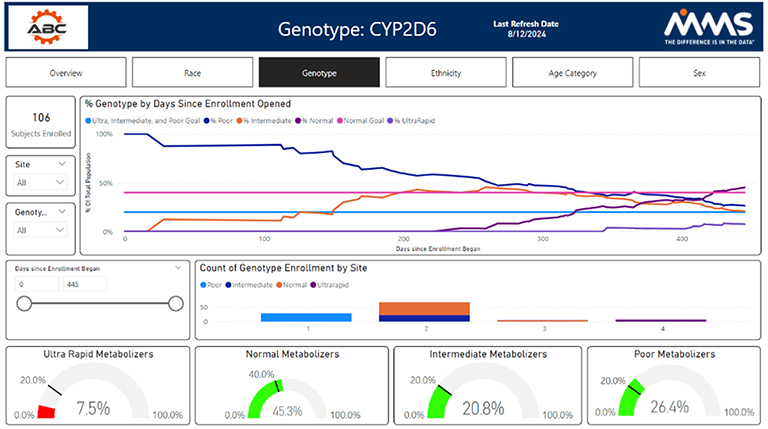
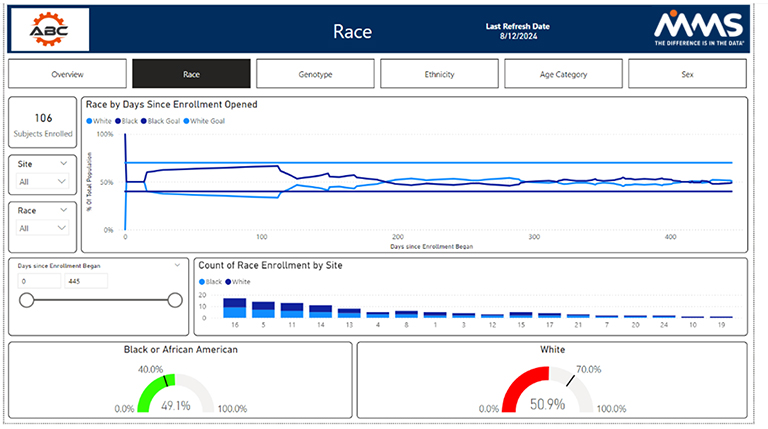
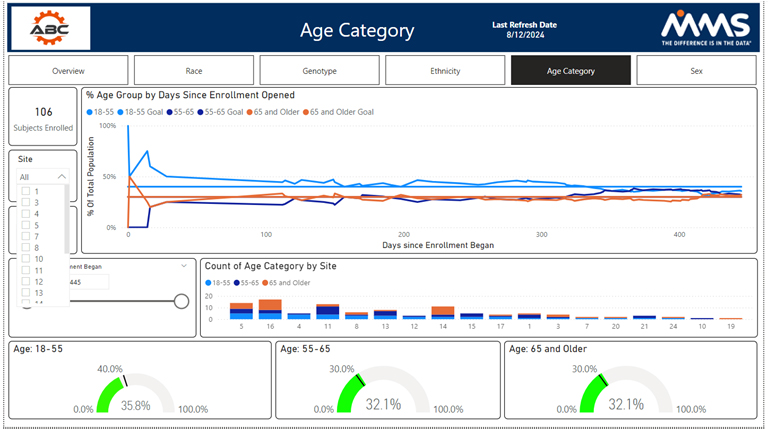
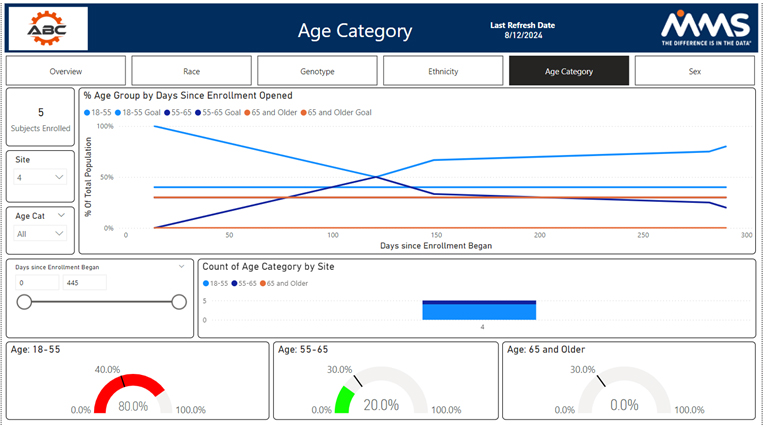
Other metrics such as race, BMI, different genotypes, age groups, and disease status are also available. The study overview dashboard seeks to summarize demographic and disposition data statuses. It is broken down at a subject, site, a by diversity category.
Visualizations are presented to highlight each diversity metric; by clicking on a visualization, a user can gain helpful insights and drill into their data. All filters, presented on the left-hand side, allow users to drill down into their data by any category of their choosing. The other examples track each metric on an individual basis by tracking enrollment by study day, enrollment by site, and whether the metric aligns with the assigned target. MMS can modify and optimize these dashboards to best fit the clinical trial at hand.
Diversity Overview
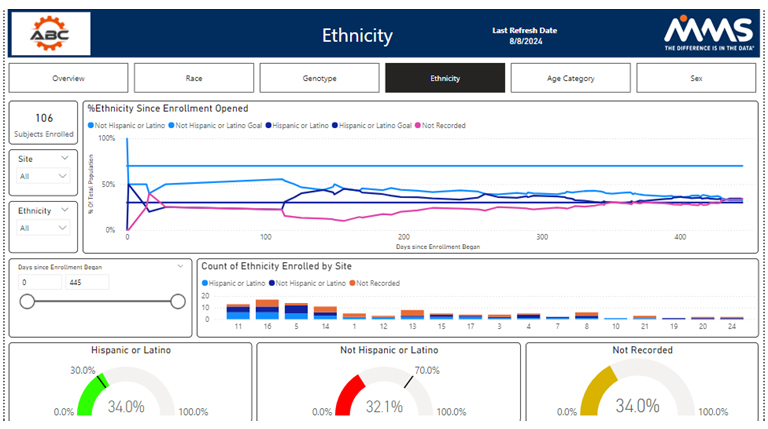
Diversity by Ethnicity
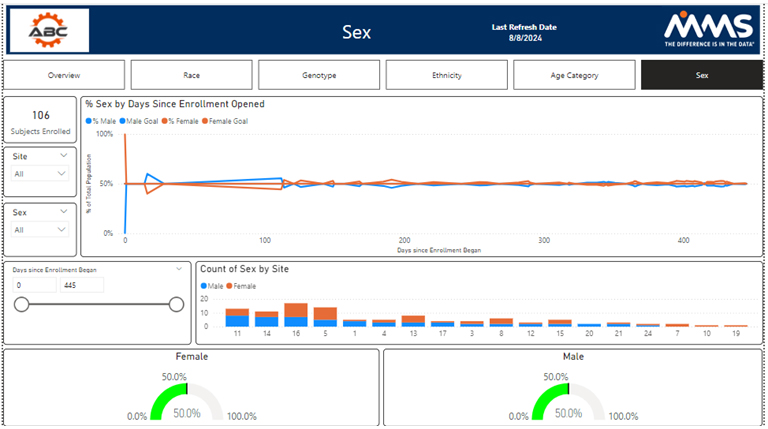
Diversity by Sex
What is the Datacise Platform?
Datacise is a browser-based data visualization tool developed by MMS to showcase interactive dashboards for Sponsors. The platform is easily accessible via an internet browser and does not require its users to acquire specialized licensing or learn how to utilize complex software. The design of the system creates secure logins for each user to ensure controlled access to available study data.
With MMS being both the developer and host of the Datacise platform, there are near limitless customizations options available. In most cases, dashboard sets can be available for viewing 2-3 weeks after the database is locked. Common uses of Datacise include, but are not limited to:
- filtering across data domains,
- combining and creating relationships between multiple datasets,
- geospatial disease prevalence,
- drug safety,
- query management, and
- statistical analysis.
Scenarios: Dispositions Mid-Trial
Post-enrollment, a subject’s attitude and/or availability to adhere to a clinical trial may change. While the initial patient enrollment numbers may fit into the regulations of the study drug, if enough subjects are discontinued or no longer comply with the clinical study protocol these numbers can shift quickly.
With Datacise diversity tracker dashboards, the diversity values can be monitored in real-time and on longer-term scales. Rather than trying to find new subjects at the end of the trial, using the diversity tracking tool allows Sponsors to be proactive and see under and over enrollment in real time.
Upon logging into the system, values falling away from acceptable tolerances will be highlighted in bright red. Additionally, email alerts can inform Sponsors of a potential enrollment issue without logging in.
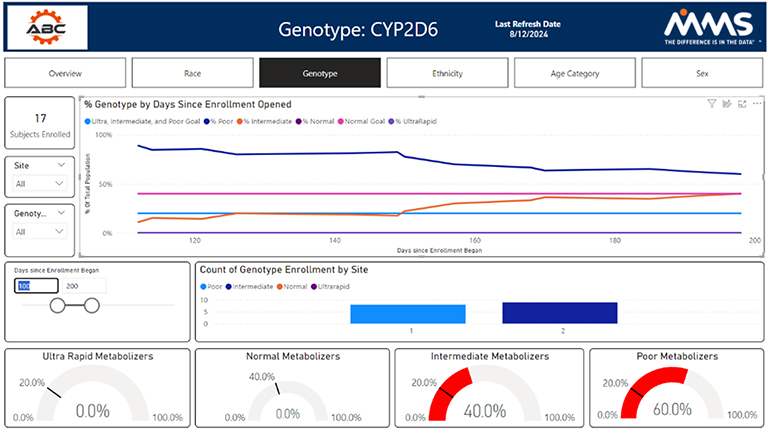
Site Selection
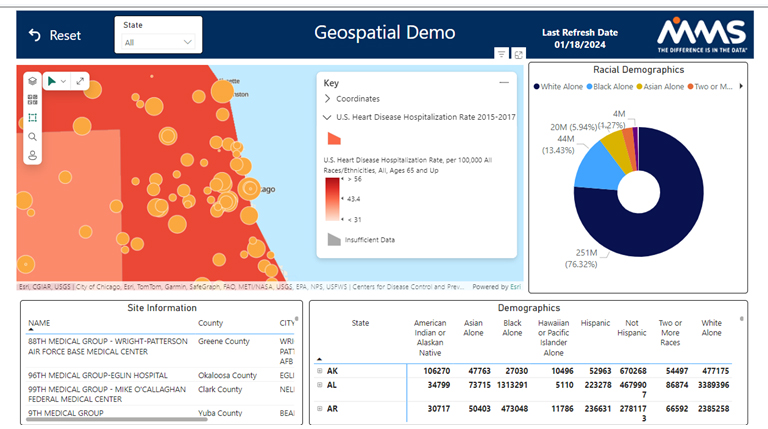
Representative sampling is imperative under the new FDA guidelines to ensure absolute drug safety. Therefore, clinical trial sites must be placed in diverse areas while also enrolling a diverse population at that location. Here, the Datacise geospatial dashboards and diversity tracker can be utilized together.
The geospatial dashboard overlays metrics such as race, gender, and disease prevalence with site location. This ensures that the right population exists at each site location. Then, utilizing the diversity tracker dashboards, the actual real-time enrollment by site can be monitored.
These two packages combined, help ensure diversity of the clinical trial in pre-enrollment, during trial conduct, and post-trial analysis. More information on the geospatial dashboard capabilities can be found here: https://www.mmsholdings.com/combining-geospatial-demographic-data-diverse-clinical-trials/
Longitudinal Analysis
In previous trials, diversity may have been managed incorrectly or in different ways before the FDA’s new guidance. Multiple studies can be aggregated together in Datacise to gain insights into how diversity has differed over multiple trials and the impact on outcomes. This information can be used to improve future study designs and to further emphasize the importance of clinical trial diversity.
Conclusions
At MMS, we have developed specific tools to operationalize the ongoing assessment of enrollment relative to Diversity Action Plan benchmarks. Combining patient disposition tracking, site selection, and longitudinal analysis with flexible and intuitive visual readouts, Datacise opens to door to iterative monitoring and rapid adjustment of Diversity Action Plans. These powerful tools and processes will help provide sponsors and investigators with the feedback they need to successfully enroll representative patient populations, ultimately leading to the approval of better therapies for all patients with unmet need.
Learn more about Datacise at www.datacise.com
This article was written by
Luke Ely, Business Intelligence Analyst II,
Amanda Beaster, Director, Regulatory Strategy,
Ben Kaspar, Regulatory Affairs and Strategic Consulting Senior Director at MMS Holdings











