How to Create Efficiencies When Creating Simultaneous NDA and MAA Submissions for the FDA and EMA

Sponsors face difficult decisions when planning for global submissions based upon available team resources and variable regional requirements. Developing each submission separately and independently for each health authority requires a considerable investment in time, effort, money, and resources.
When submitting in the EU, US, or other regions in parallel, a streamlined approach to drug approvals is recommended to ensure the process is efficiently optimized for resource allocation and is feasible with proper planning at the onset of submission preparation. Achieving such efficiencies first requires an understanding of the key differences and similarities between different regulatory submissions. This eliminates rework and creates clear, direct development of the proper path from US to EU submission or vice versa. In this Perspectives article, the focus will be on marketing applications to both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Agile and efficient strategy execution at every stage including clear messaging to describe the results of the clinical and nonclinical development programs, product development rationale, therapeutic context are important, to maximize the strength of the risk-benefit argument and to minimize queries and information requests post submission. CROs can implement a Global Submission Strategy and collaborate with the Sponsor to optimize content reuse while creating the lead application and generating subsequent submissions to other regional health authorities.
For instance, it is best practice to develop clinical key messaging ahead of database lock for the Phase 3 Clinical Study Report (CSR) – preferably prior to or in tandem with the CSR shell – so that key messaging is consistently interwoven throughout each module of the dossier.
A thorough gap assessment with a cross-functional team, including experts in data, biometrics, and regulatory strategy who can conduct a review of prior submission/commitments, files/documentation, data, and agency interactions, can provide an ease at the time of developing a global dossier that supports multi-regional applications and highlight topics for discussion with both regulatory agencies.
How to Reduce the Number of Drafts
From document authoring to compilation, extensive collaboration is required to streamline workflows, enhance cross-departmental efficiency, and concurrently develop the FDA and EMA applications to ensure seamless submissions. During authoring, staggering draft reviews when producing multiple deliverables with inter-related content allows for efficient reuse of content across documents. This technique can reduce the number of drafts and reviews required.
Best practice when working on global dossiers is to minimize or eliminate regional differences wherever possible in the eCTD. This can help reduce effort in preparation of each subsequent submission and ease lifecycle maintenance activities post-submission. An experienced global submissions team who is well aware of the different regulatory requirements can pivot as needed to navigate the complexities of regulatory submissions.
Developing each submission separately and independently for each health authority requires considerable investment in time, effort, money, and resources. Achieving such efficiencies first requires an understanding of the key differences and similarities between different regulatory submissions.
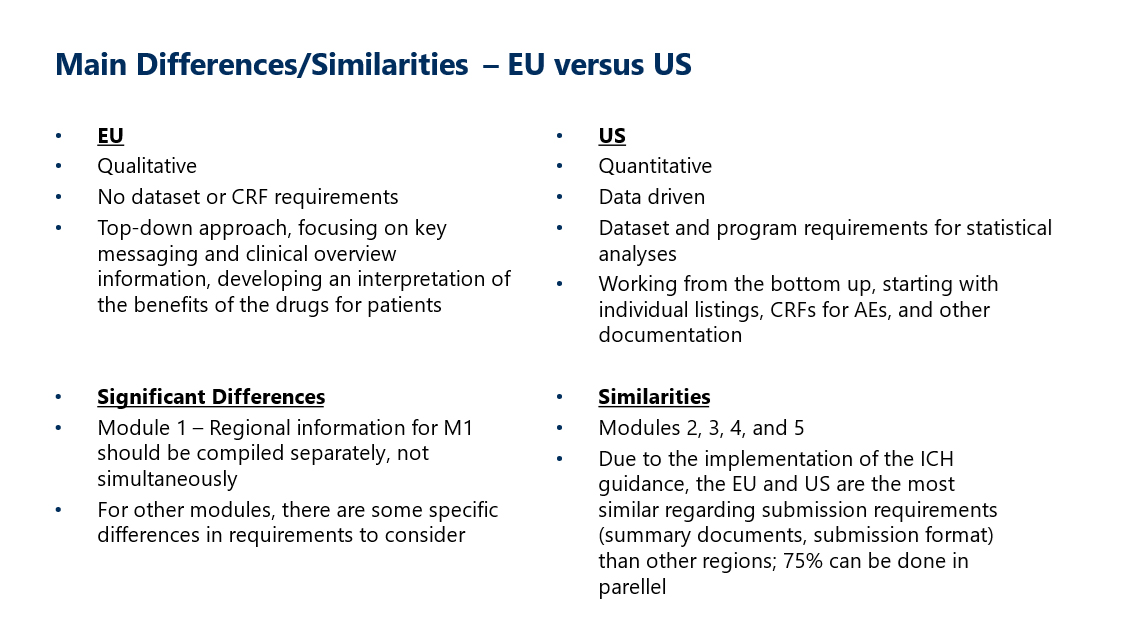
Key Similarities and Difference Between US and EU
Both FDA and EMA submissions are science-driven and focus on robust clinical data to ensure patient safety and efficacy of the drug product. They follow the CTD format for submission modules covering quality, pre-clinical and clinical data, which allows for simultaneous submissions across multiple health agencies.
Although the requirements for eCTD are similar across EU and US, there are still significant areas to consider that may need specialized attention regarding what is required per document per region. Some of these considerations include terminology, datasets, STFs, and use of document/submission metadata. It is beneficial for submission teams to document these differences in a checklist to align on the best approach for planning and collaboration to ensure success.
Modules can be written and repurposed for various global submissions, with insight into regional priorities. A strong scientific justification in Module 2 for an NDA can be updated with scientific advice given by the EMA for a Marketing Authorization Application (MAA). With multiple meeting types, it is important to work with a regulatory strategist or medical writer who can develop clear and concise briefing documents that align with the sponsor’s goals and timelines.
Differences in Health Authority submission review timelines should be considered with emphasis on the applicability of any accelerated/expedited review pathways. FDA standard timelines are 10 months versus EMA centralized procedures, which can last up to 210 days, or around 7 months.
Accelerated/expedited pathways will further shorten these timelines for qualified drug development programs. FDA priority review reduces timelines from 10 months to 6 months while EMA accelerated assessment usually reduces assessment time from 210 days to 150 days, or around 5 months. Both EMA and FDA offer scientific advice throughout the drug development process and prior to submissions. The EMA conducts these in the form of Scientific Advice or protocol assistance, while for the FDA it is only offered to drugs that are granted orphan drug products.
Regulatory leads should be aware of US- and EU-specific CMC guidance for drug substances, drug products, and stability data, and their historical knowledge of the program will help teams navigate agency queries. The FDA may reach out with information requests at any time and will assign a timeframe in which a response is expected. For the EMA, they will provide initial assessment reports and a list of questions at 120 days to which the Sponsor will have to respond, following a clock-stop that usually lasts three months.
Having a robust regulatory plan and global experienced team is essential for seamless submissions to the FDA and EMA.
Best Practices for Aligning EMA and FDA Submissions
To properly align submission to both global health authorities, consider the following:
- Elaborate planning at the start of the documentation process helps alleviate issues and optimizes submissions.
- Build a thorough understanding of the requirements of FDA and EMA, their timelines, and the content that can be common across both submissions at the start of the documentation process.
- Understand the documents that are mandated or required for one of the health authorities and is not explicitly required for the other. Identifying the documents that are not mandatory for either one of the health authorities saves time and effort by avoiding development of unnecessary documents. For instance, the FDA requires ISS (integrated summary of safety) and ISE (integrated summary of efficacy) to be included as separate sections withing Module 5 of the CTD; however, these documents are not mandated by EMA, where the safety and efficacy data is included in Module 2.
- Ensure the data, wherever possible, is in alignment for both the FDA and the EMA and alter the way of presentation where required for one of the submissions.
- Keep in mind the differences and nuances between US English (for FDA) and UK English (for EMA).
- If possible, the Sponsor should outsource the preparation of the NDA and MAA submissions to the same Contract Research Organization (CRO) to ensure consistency in data presentation.
In order to avoid pitfalls when building multiple regional submissions, Sponsors should:
- Always consider multiple submission timelines when planning global submissions. Some regions follow agency-mandated timelines, which may dictate parallel submissions or sequential builds.
- Ensure the key messaging, terminology, and the documentation is optimized for multi health authority submissions from the onset of NDA discussions.
- Start with the end goal date and work backward to determine how far in advance each discussion needs to occur and identify the timeline for completing each task.
- Begin by creating a master outline that includes common documents. Use an advanced eCTD tool to clone submissions for multiple regions, ensuring efficiency and minimizing rework. After cloning, add or edit country-specific documents as needed (e.g., add STFs for US NDAs).
- Implement a dual-lead approach, assigning each lead to focus on specific modules for both EU and US regions. This approach ensures clear responsibility, enables focused development of respective modules, and maintains accountability for quality control and consistency across regions.
These best practices ensure that both submissions are developed with maximum efficiency and minimal effort, delivering a consistent package tailored to country-specific requirements within the shortest possible timelines.
Multiple or simultaneous submissions for different health authorities are effective ways to save time, money, and effort to the Sponsors and also to ensure that the patients in need get quicker access to the medications. By understanding and leveraging the similarities between submissions, Sponsors can navigate these submissions successfully and get approvals to drugs and save millions of lives across the globe.
This article was authored by
Tejaswini Ravindranath, Associate Manager, Regulatory and Medical Writing
Jami Gentry, Technical Manager, Regulatory Operations
Raksha Srinivas, Team Lead, Regulatory Operations
Sanjana Sheth, Principal Regulatory Strategis, Regulatory Affairs
For questions related to the content within, email info@mmsholdings.com.
References:
Simultaneous New Drug Submissions: Is it Possible?
Development & Approval Process | Drugs | FDA
Procedural timetables | European Medicines Agency (EMA)











