Clinical Trial Design and Analysis
Harness next-generation cloud-based study simulation for unique statistical insights supporting earlier decision-making and more successful clinical development.
The KerusCloud® platform has proved to be a core part of our decision-making as it allows us to prospectively tailor study designs as new information becomes available.
Smarter Clinical Trial Design and Analysis with KerusCloud®
KerusCloud® is a powerful clinical trial design and analysis software that enables sponsors to simulate thousands of realistic trial scenarios in silico. By modeling variables such as patient populations, endpoints, recruitment timelines, and estimand strategies, KerusCloud® helps teams identify the optimal design before a trial begins. Fast, data-driven, and cost-effective, it empowers you to reduce risk, accelerate timelines, and increase the likelihood of success.
Using KerusCloud®, our team helps sponsors accelerate development by identifying the right path, first time. Keruscloud® has been seen to increase the probability of success of a single study by 41% and generates more evidence for the same costs. Working in tandem with our clients, we have a track record of de-risking investment through rapid evaluation and testing of assumptions, adding value to current drug pipelines and existing data.
Our design optimization approaches are applied to:
- Target population
- Study Design
- Endpoints
- Analysis Strategy
- Success Criteria
- Selecting the best development strategy
- Choosing between standard or complex innovative design trials such as adaptive platform trials (APTs), seamless studies, basket trials and umbrella trials
- Improving study success rates
- Identifying the best endpoint and patient subgroups
- Generating robust evidence packages for drug approval
- Supporting investment decisions
- Implementing Precision Medicine strategies
- Anonymizing real-world data by generating realistic synthetic data
Transform Traditional Study Design with KerusCloud®
KerusCloud® facilitates informed decision-making by placing statistics at the heart of protocol development, addressing the growing complexity of modern clinical studies.
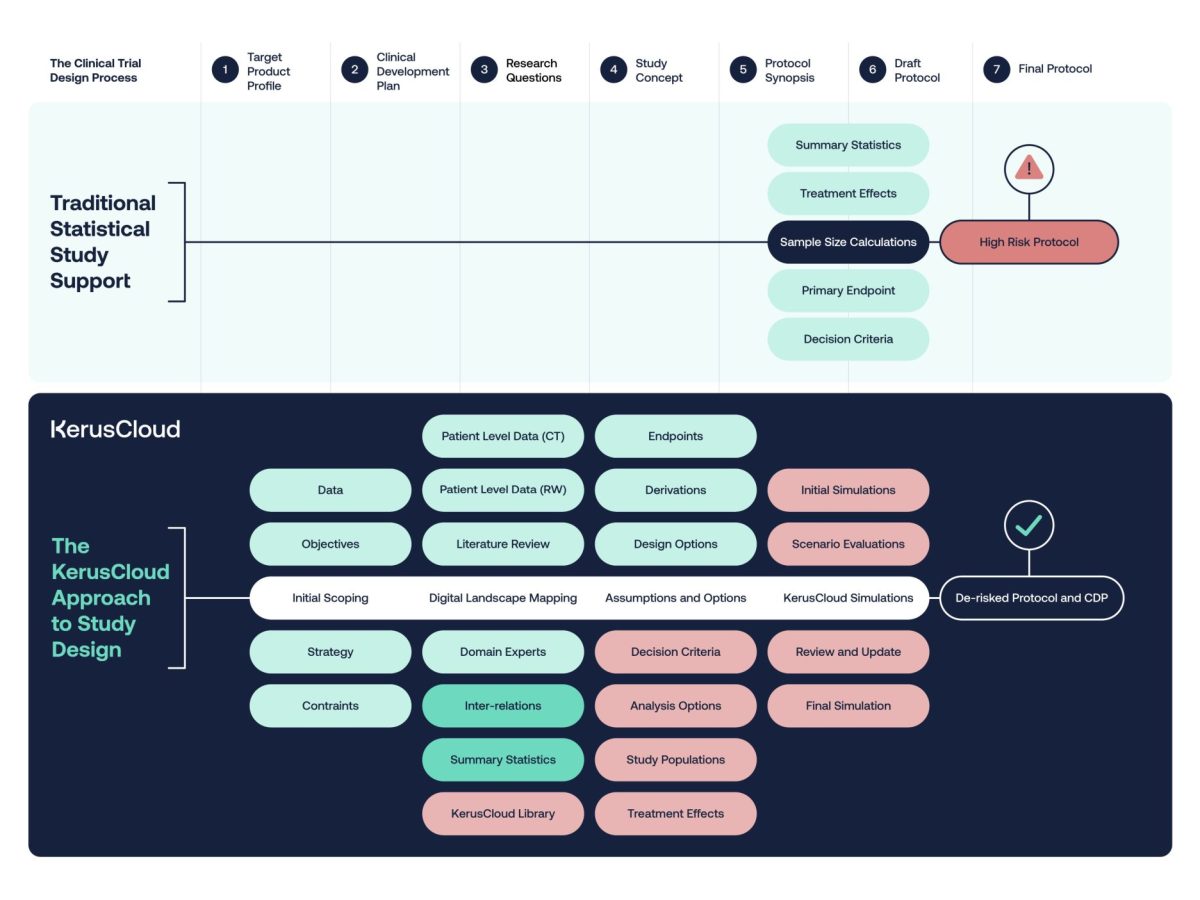
By incorporating KerusCloud® into the clinical protocol development process information can be leveraged from available data earlier to inform key decisions. This ensures that real clinical studies are comprehensively de-risked and fully optimised in silico at the design stage using its rigorous evidence-based approach.








