Pulmonary Disease Specific Data Models
Informing respiratory study assumptions with pulmonary research data
KerusCloud® simulations are very intuitive. You can have a quick understanding of what the goals are, what the outputs could be, and it enables quick decision-making from the whole team regardless of their statistical background.
De-risk Respiratory Clinical Trials with Pulmonary Data Models
KerusCloud® Pulmonary Suite equips your team with expertly curated clinical trial data models across a wide range of respiratory conditions—from COPD and IPF to asthma and RSV. Designed by our Data Science team using real-world, regulatory-grade datasets, the suite helps sponsors and researchers identify optimal endpoints, biomarkers, patient populations, and design strategies. Whether you’re optimizing a Phase II study or validating assumptions for regulatory submission, KerusCloud® accelerates decision-making and reduces trial risk—before your study ever begins.

The pulmonary suite provides reliable, ready-to-use historical data across a range of pulmonary indications (Figure 1). Offered at different complexity levels according to project demands, the data models give an extensive overview of the pulmonary clinical trial landscape, clinical outcomes, and patient characteristics. This information can then inform study simulations in KerusCloud® to support evidence-based clinical trial design.
Data Science Services can sift through information relating to thousands of clinical trials in the suite, identifying relevant historical data to inform the planning of your study. The suite can be searched by:
- Disease
- Endpoint
- Patient population
- Biomarker
- Interventions
- Phase
- Enrolment
- Design
Selected information can be built into realistic synthetic patient-level datasets for use in running virtual clinical trials in KerusCloud®. Trial scenarios of interest can be simulated, and their outcomes examined to answer key ‘what if’ questions relating to study design and analysis. This ensures you identify, with confidence, the right approach for your pulmonary asset in a real clinical trial.
Pulmonary Suite
Complexity Level Overview
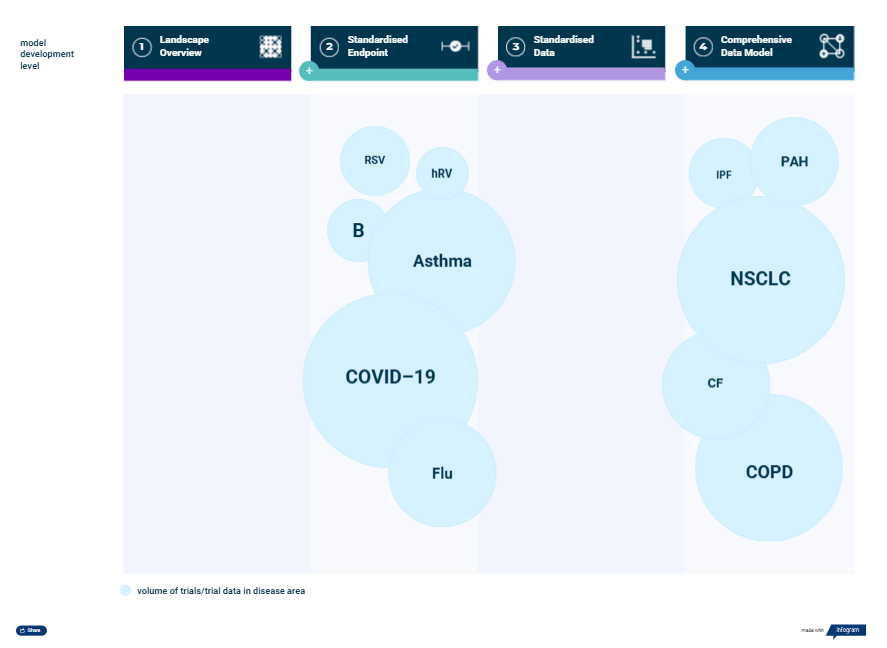
Figure 1. Disease models included in the Pulmonary Suite are available at four levels of complexity/granularity: 1) landscape overview, 2) standardized endpoint, 3) standardized data and 4) comprehensive data model. The indications included are pulmonary artery hypertension (PAH), idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), asthma, COVID-19 (CV19), bronchiectasis (B), influenza (Flu), non-small cell lung cancer (NSCLC), rhinovirus (hRV) and respiratory syncytial virus (RSV).








