Essential Nonclinical Strategies for Cell and Gene Therapy (CGT) Success
Cell and gene therapy (CGT) or advanced therapy medicinal products (ATMPs) – FDA’s and EMA’s terms, respectively – have been an emerging field for the past few decades, because of their potential in treating chronic diseases, inherited diseases, and for providing new treatment for prevalent diseases. However, product launches have been slow to progress, due to the controversial landscape that surrounds the high costs, ethics, access and equity, regulatory framework, long term safety and the experimental therapies in the CGT field.
In 2023, there was a breakthrough of CGT products, with 7 products approved by the FDA1 and 1 approval by the EMA, paving the way for a phenomenal number of CGT approvals expected in the next year and beyond.
Cell and Gene Therapy Attributes
Although often used in the combined form of ‘CGT’, cell therapy and gene therapy are distinct when not used in tandem. Essentially, cell therapy uses whole functioning cells modified with the relevant function, whereas gene therapy involves altering the genetic material and transfers it into a cell type that is administered back into the body via a delivery carrier. Both cell therapy and gene therapy are broad technologies and can be split further into many sub-categories.
The characteristics of CGTs are generally considered more complex than those of traditional modalities, such as small molecule pharmaceuticals and biopharmaceuticals. Table 1 below highlights some of these challenging aspects, that in turn will require more refined technical and medical writing input.
Table 1: Product Attributes of Cell Therapy and Gene Therapy in Comparison to Small Molecule Pharmaceuticals and Biopharmaceuticals2
NHP = non-human primate; PD = Pharmacodynamic; WT = Wild Type
* Cellular and humoral responses – the potential for the immune system to react to the therapeutic cells/vectors, leading to safety concerns.
Innovative Nonclinical Attributes of CGTs
In addition to the standard attributes applicable to other modalities covered earlier, CGT also includes continuously evolving features that consist of improved nonclinical models, biomarker development, and novel toxicology approaches. Examples of innovative attributes include:
- Delivery3: This refers to the vehicle or vector (non-viral or viral vectors) that the desired gene in the targeted cell is delivered back into the body. This can also involve encapsulation technologies or novel devices. Since many key organs remain difficult to reach with this technology, this is one of the attributes that continues to evolve.
- Prophylaxis/De-immunizing/Tolerization Strategies: These attributes provide valuable insights in the safety, tolerability, and immunogenicity profile of CGT products.
- Payload: Understanding and optimizing the active ingredients is crucial in conveying the design of CGTs. This can include on/off “switches”, intracellular and extracellular proteins, etc.
- Route of administration (ROA): CGT offers the most diverse routes for any modalities and can include dual ROAs as well as novel devices.
Regulatory Landscape for CGTs – FDA and EMA Guidelines
There is a plethora of guidance on CGTs between the two of the major regulatory agencies, the US Food & Drug Administration (FDA) and the European Medicines Agency (EMA). Of those, there are two key detailed sets of guidance that provide specific guidelines around expectations for nonclinical studies prior to the initiation of clinical studies for CGT products:
- Preclinical Assessment of Investigational Cellular and Gene Therapy Products | FDA4, and
- Quality, preclinical and clinical aspects of gene therapy medicinal products – Scientific guideline | European Medicines Agency (europa.eu)5.
The EMA guidance also references a separate guideline on the risk-based approach applied to ATMPs6.
To summarize, the FDA guidance focuses on the design and conduct of nonclinical pharmacological and toxicological studies with emphasis on the specific product characteristics, whereas the EMA guidance looks at the nature and extent of the nonclinical development by the attributes of the gene therapy medicinal products (GTMP).
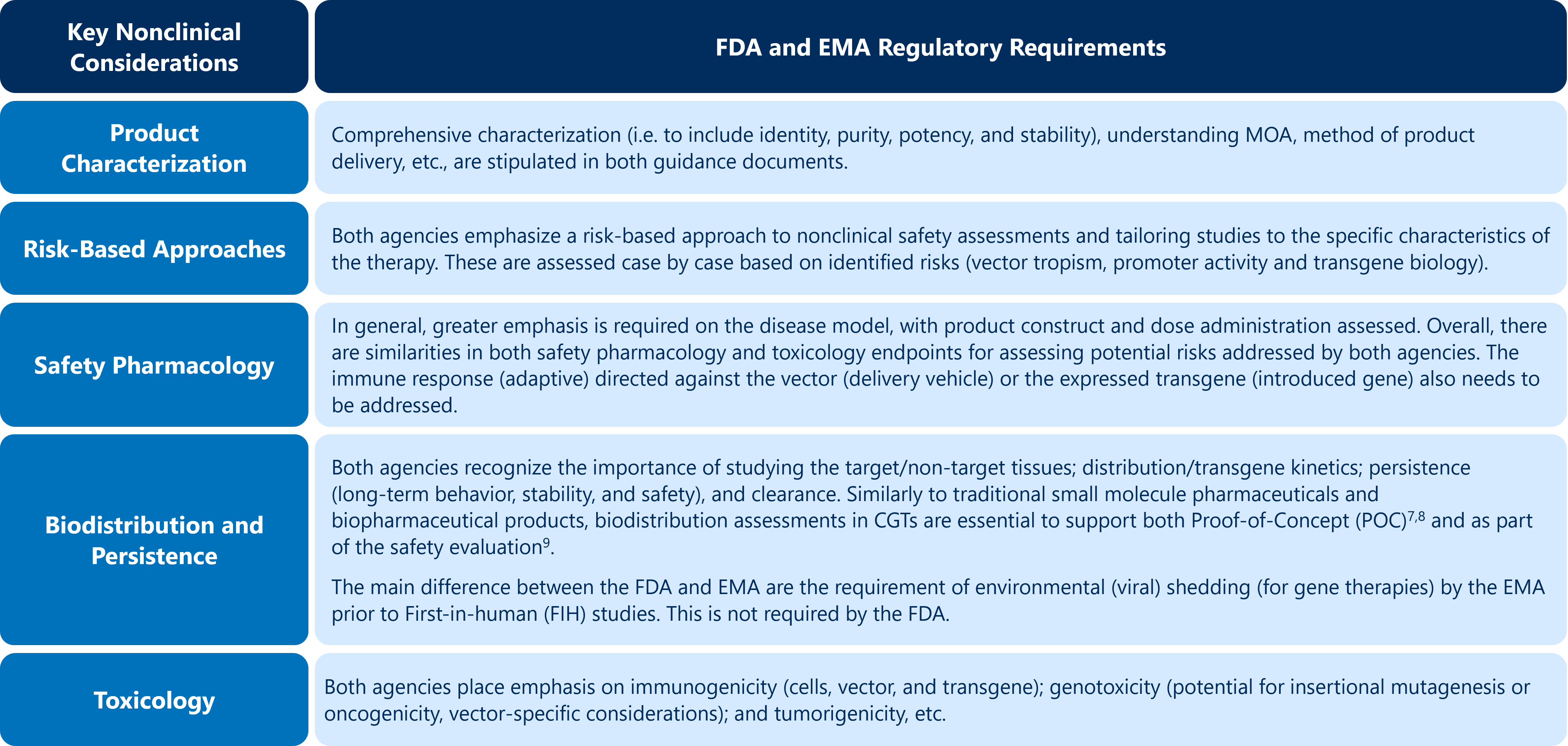
The nonclinical principles are essentially the same for CGTs compared with other modalities. However, both regulatory authorities place high value on several key points in the guidance, as outlined in Table 2 below. Failure to appropriately address these points can result in delay of a CGT program.
Table 2: Key Nonclinical Considerations for CGTs in Relation to the Key FDA and EMA Guidance
The FDA’s guidance recommends maintaining strong relationships and interactions with regulatory agencies. Sponsors should engage early with the Office of Therapeutic Products (OTP) INTERACT (Initial Engagement for Regulatory Advice on CBER Products) group, in addition and prior to the
pre-IND meeting. Although the EMA does not have an exact equivalent to the FDA’s INTERACT meeting, the EMA encourages Sponsors to seek feedback through the EMA’s scientific advice service.
Important Considerations for Authoring Nonclinical Regulatory Documents for CGTs
CGTs regulatory submissions require specialized background and expertise when summarizing nonclinical development programs, and the following should be considered when authoring nonclinical submission documents for CGTs:
- Complexity of science: Due to the intricate nature and the multidisciplinary approach involving molecular biology, genetics, and immunology, a deep understanding of the scientific aspect is required to ensure accurate and comprehensive documentation.
- Continuous learning: It is essential to stay abreast of the latest advancements and industry trends, including those from the relevant committees and working groups like The Cellular, Tissue & Gene Therapies Advisory Committee (FDA), the Committee for Advanced Therapies (CAT) (EMA), and The International Pharmaceutical Regulators Program (IPRP) for Gene Therapy Working Group (GTWG) initiatives.
- Regulatory experience: The regulatory landscape for CGT products is dynamic and can be confusing, thus a good grasp of the regulatory requirements and guidelines in nonclinical space is essential to provide a cohesive and data-focused overview of nonclinical program for CGTs.
- Lack of standardization: This includes the absence of animal models of disease/non-standard study design/methodology/deviation from the default animal models, etc. Adaptive medical writing practices and the articulation of complex concepts to convey sound scientific rationale are thus required.
- Addressing current/novel nonclinical challenges: This involves identifying issues in nonclinical models (predictive, in vitro, in vivo, etc.). These challenges cover immunogenicity, genotoxicity, and the potential for uncontrolled proliferation, just to name a few.
- Risk assessments: A comprehensive risk-based approach is required to effectively convey the potential risks associated with CGTs, with clarity, transparency and in a scientifically-accurate manner.
Another separate, yet equally important aspect in the development of the preclinical package for CGT also includes chemistry and manufacturing controls (CMC), which are discussed in the following Blog post: Cell and Gene Therapies (CGT): 6 Detailed CMC Considerations for IND Submissions.
For guidance tailored to your specific needs and to help reach your CGT/AMTP’s nonclinical submission goals, please contact the MMS team.
This article was written by Wai-Fung Chau, Senior Medical Writer, Regulatory and Medical Writing
References
- Approved Cellular and Gene Therapy Products | FDA
- American College of Toxicology. eLearning Seminar. Gene Therapy: Nonclinical and Regulatory Strategy Module 1 to 4 (2023)
- Guideline on the non-clinical studies required before first clinical use of gene therapy medicinal products (EMA Nov 2008)
- Guidance for Industry: “Preclinical Assessment of Investigational Cellular and Gene Therapy Products (FDA Nov 2013)
- Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products (EMA Mar 2018)
- Guideline on the risk-based approach according to Annex I, part IV of Directive 2001/83/EC applied to Advanced Therapy Medicinal Products (EMA Feb 2013)
- Reflection paper. Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products (IPRP Apr 2018)
- ICH S12 Nonclinical Biodistribution Considerations for Gene Therapy Products (FDA May 2023)
- Guidance for Industry: long Term follow-up After Administration of Human Gene Therapy Products (FDA Jan 2020)











