REMS Logic Modeling: Applying FDA Guidance from November 2024 CDER Webinar
The Small Business and Industry Assistance (SBIA) program in the Center for Drug Evaluation and Research (CDER) hosted a highly informative webinar on November 7, 2024. This session, Putting the Pieces Together: REMS Logic Model in REMS Design, Implementation, and Evaluation, reviewed the benefit of logic models, as documented in the FDA’s draft guidance document published in May, 2024.
With this guidance, the FDA provides sponsors with a path to think strategically while adopting an iterative approach throughout REMS design and implementation.
For those who may be less familiar, Risk Evaluation Mitigation Strategy (REMS) is a drug safety and risk management program established in 2007 by the U.S. Food and Drug Administration (FDA). With each REMS having different requirements, REMS design typically begins with the REMS elements, materials, and timetable in mind. The chosen method at MMS includes establishing the safe-use and assessment reporting goals upfront to identify key aspects before any design commences.
Leveraging the REMS Logic Model to define a REMS program is a natural progression of our approach to REMS implementations, and the goal for ongoing safety and continuous improvement.
In principle, logic models are simply visual roadmaps to join the design, implementation, and outcomes of a planned project. With the FDA’s utilization of logic models for REMS, the linear view provides one representation of the steps sponsors need to consider, as seen in Figure 1 below.
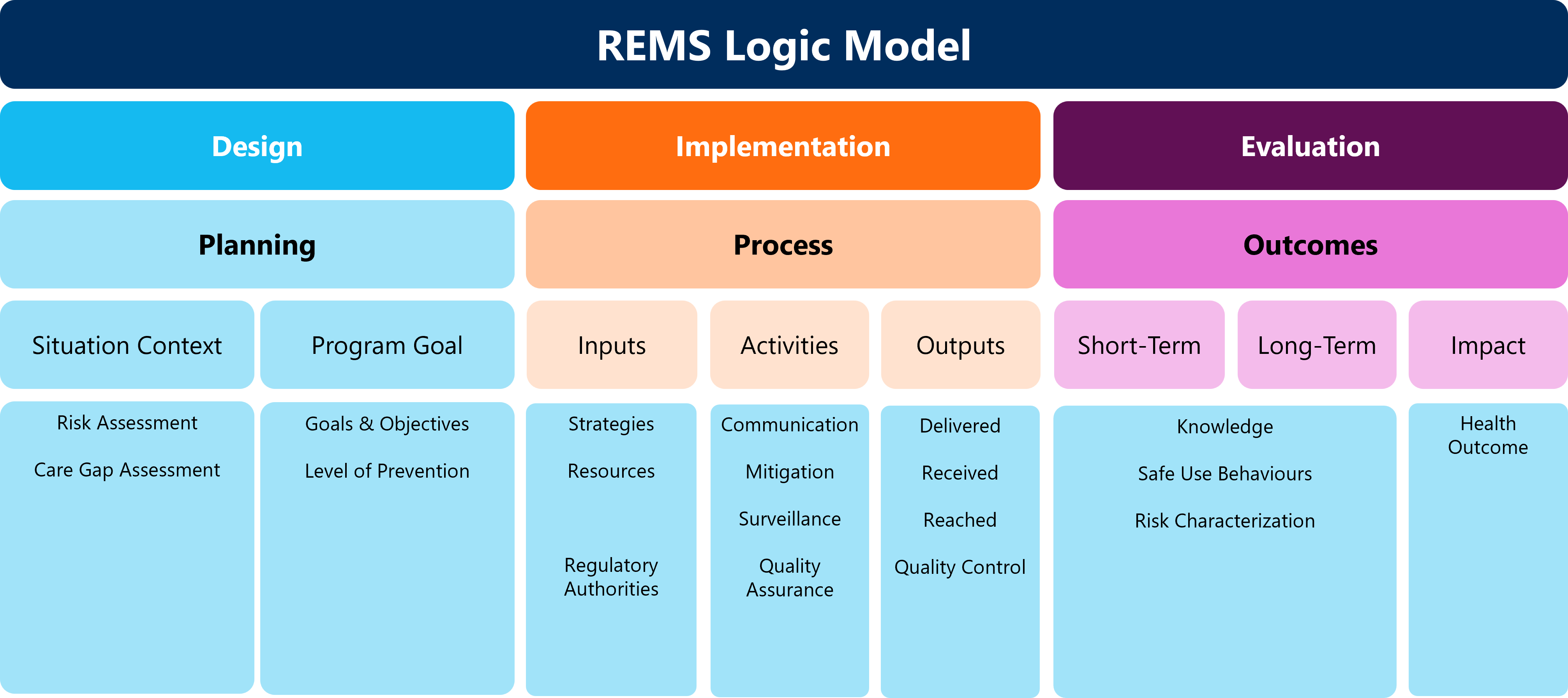
Key considerations are necessary to ensure the logic model effectively guides program design, implementation, and evaluation.
Designing REMS Logic Models
Planning activities must consider the situational content of the product. Begin by conducting an in-depth Risk Assessment of the serious risk(s) that must be mitigated, including the following elements:
- Review preclinical and clinical data, literature evaluation, post-marketing data, epidemiologic studies, and real-world data.
- Known potential SAEs and severities, frequency, details of administration, and patient related factors.
- Knowns, unknowns, and assumptions should also be identified during this assessment.
Then, conduct a Care Gap Assessment that involves identifying discrepancies between the data that you have reviewed and best practices of care that can be mitigated by a REMS, including the following elements:
- Baseline knowledge.
- Attitudes of patients.
- Behaviors of prescribers.
- The anticipated medication use process.
The activities conducted during the Risk and Care Gap Assessments will organically lead to identifying potential problems and prevention for program goals. The objectives desired by the REMS include the primary prevention of a serious adverse event from occurring, as well as early identification to prevent the event from worsening. Tertiary prevention can also be identified through this exercise and establish what patient monitoring could do to improve event prevention.
Identifying different preventative actions and their levels will allow you to recognize critical benefit-risk considerations and communications that must be shared with prescribers and patients.
While still in the early design phase, the above activities can yield initial key performance indicators (KPIs) that are critical to any REMS, measuring the goals and objectives to safe-use.
Implementation of REMS Logic Models
Identifying what processes will drive your REMS can be defined by detailing your Inputs, Activities, and Outputs. Inputs are identified by determining what you need to operate your program. This includes analysis of strategies, resources, and, as the design is defined, engagement with regulatory agencies like the FDA.
REMS strategy includes addressing what affects knowledge and behavior, and inform of risk mitigation. It also includes identification of the tangible aspects, including those communication strategies to improve knowledge, like Medication Guides, Communication Plans, Training, and the possible certification of stakeholders.
The strategy is further refined by identifying the mitigation strategies, where you identify such REMS rules as verification before dispensing, documentation of safe use, and possible monitoring via observation or lab results. Your strategy should also include any surveillance approach to inform risk mitigation, such as a patient registry.
As the strategy begins to take shape, you will identify the resources that are required. Resources are inclusive of people involved in and/or participating in your REMS, the materials such as communications and enrollment forms required, and the critical technology necessary to ensure the REMS is available to all critical participants.
Activities in the implementation of REMS Logic Models are defined as the actions of a program to achieve the program objectives. These will equate to REMS requirements and will be defined in the REMS Document. REMS activities can be broken down in three categories:
- Communication
- Mitigation
- Surveillance
Based on what you have determined your inputs to be, activities can be easily identified. Like anything, your input will determine what you get out of your REMS.
Outputs in the implementation of REMS Logic Models are the results of the activities implemented and it measures how the REMS is operating. The following outputs should be included:
- Restrictions on dispensing within a REMS platform and confirmation that mitigation activities were delivered.
- Confirmation of training by stakeholders to verify that communication has been received.
- Enrollment by relevant stakeholders provides output that your strategy goal has been reached.
Evaluation of REMS Logic Models
The final aim of REMS logic modeling is Evaluation. This begins with defining the outcomes which are derived from the outputs from implementation.
Outcomes provide insight into the impact and health outcomes that we expect to get out of the program. Measuring impact, such as using test results after REMS training, will confirm education goals. This analysis can also ensure identification of any risks that were overlooked or resources that require additional changes to reduce health impacts, for instance.
Defining the outcomes and impact as it links to the Design strategy as well as the Implementation inputs and outputs will provide a holistic evaluation. If this does not occur, the logic model will more than likely guide you to where change is needed.
The value of ongoing analysis throughout each phase of logical modeling in REMS can identify and mitigate risk that may have been previously overlooked. The CDER-hosted webinar further recapped the invaluable worth of logic framework: success must be defined before it can be achieved.
It was reiterated during the webinar that this is guidance, not a requirement. However, the FDA is open to logic models being included in a sponsor’s REMS Supporting Document.
Email info@mmsholdings.com to be connected with the author or for more information on how MMS can enhance your REMS submission with logic modeling.
This article was written by Christine Manley, REMS Director, Pharmacovigilance at MMS.











